Our pharmaceutical grade facility is designed to comply with AU and EU GMP (Good Manufacturing Practice) standards. The processing part of the facility is designed to align with the TGA’s GMP benchmarks.
Key areas of GMP compliance affecting the GEMKOM facility include:


Good Manufacturing Practice (GMP) ensures products consistently meet safety and quality requirements. It is based on monitoring and assessing compliance with the 5Ps: Premises, People, Procedures, Product and Processes.
GMP is globally recognised and preferred by suppliers of food, medicine, and therapeutic goods.
Australian GMP requirements for medicinal marijuana are based on the requirements of the EU guidelines.

To maintain GMP certification, firms are regularly audited and assessed, by internal teams and, crucially, third party certification bodies including the TGA.
The elements of a GMP system are complex and involve all practises that can impact quality or safety, from equipment/facility hygiene (to control microbiological risks) to traceability and management controls.
Additional examples of GMP elements include:

Though some regard compliance as a risk (or negative), GEMKOM understands that in both the medical and recreational cannabis space GMP is mission critical, not simply for licensing but for commercial and ethical reasons.
GMP compliance underlines trust all along the supply and value chains – and trust is the core ingredient when producing goods that people consume as medicine, food or smokables.
In addition, GMP metrics serve to drive best practise, providing measurable benchmarks by which to measure and track performance across the business.

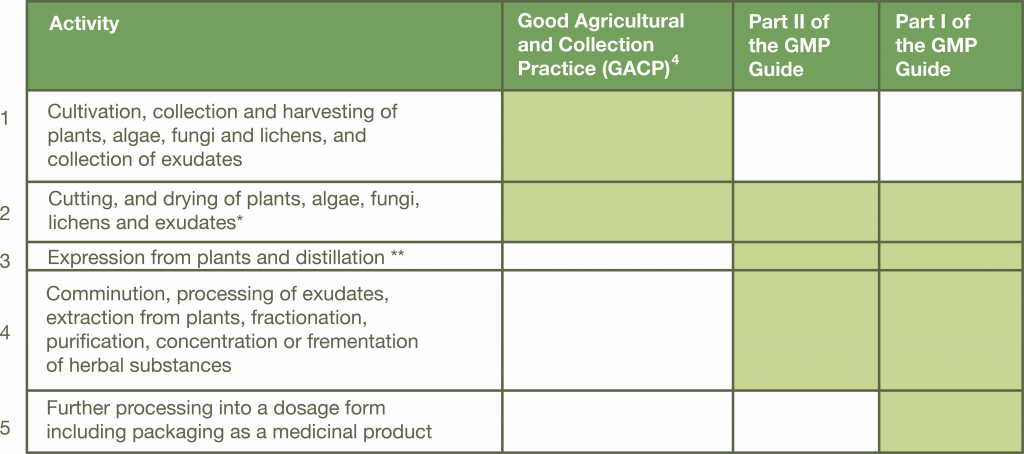
Our facility and processes comply on all five activity points outlined in the GMP guidelines and will cover both GACP (Good Agricultural Collection Practise) and Part I & II of GMP guide. Our end product (dry flowers) will be considered medicinal substances for products in final dosage form and final packaging, ready for the consumer.
The table below outlines the specific certification requirements of a medicinal marijuana facility, with the scope of certification requirements outlined from cultivation to finished product. It also highlights the difference between GACP and GMP certification requirements.
Part I – Medicinal
Part II – Active Substances
*GMP Guidelines